
HL Paper 1
Given the enthalpy change for the reaction below:
\[\begin{array}{*{20}{l}} {{\text{2}}{{\text{H}}_{\text{2}}}{\text{(g)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}}}&{\Delta {H^\Theta } = - 572{\text{ kJ}}} \end{array}\]
which statement is correct?
A. The standard enthalpy change of combustion of \({{\text{H}}_2}{\text{(g)}}\) is \( - 286{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
B. The standard enthalpy change of combustion of \({{\text{H}}_2}{\text{(g)}}\) is \( + 286{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
C. The standard enthalpy change of formation of \({{\text{H}}_2}{\text{O(l)}}\) is \( - 572{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
D. The standard enthalpy change of formation of \({{\text{H}}_2}{\text{O(l)}}\) is \( + 572{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Consider the two reactions involving iron and oxygen.
\[\begin{array}{*{20}{l}} {{\text{2Fe(s)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2FeO(s)}}}&{\Delta {H^\Theta } = - 544{\text{ kJ}}} \\ {{\text{4Fe(s)}} + {\text{3}}{{\text{O}}_2}{\text{(g)}} \to {\text{2F}}{{\text{e}}_2}{{\text{O}}_3}{\text{(s)}}}&{\Delta {H^\Theta } = - 1648{\text{ kJ}}} \end{array}\]
What is the enthalpy change, in kJ, for the reaction below?
\[{\text{4FeO(s)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2F}}{{\text{e}}_2}{{\text{O}}_3}{\text{(s)}}\]
A. \( - 1648 - 2( - 544)\)
B. \( - 544 - ( - 1648)\)
C. \( - 1648 - 544\)
D. \( - 1648 - 2(544)\)
Enthalpy changes of reaction are provided for the following reactions.
\[\begin{array}{*{20}{l}} {{\text{2C(s)}} + {\text{2}}{{\text{H}}_2}{\text{(g)}} \to {{\text{C}}_2}{{\text{H}}_4}{\text{(g)}}}&{\Delta {H^\Theta } = + {\text{52 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \\ {{\text{2C(s)}} + {\text{3}}{{\text{H}}_2}{\text{(g)}} \to {{\text{C}}_2}{{\text{H}}_6}{\text{(g)}}}&{\Delta {H^\Theta } = - {\text{85 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
What is the enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the reaction between ethene and hydrogen?
\[{{\text{C}}_2}{{\text{H}}_4}{\text{(g)}} + {{\text{H}}_2}{\text{(g)}} \to {{\text{C}}_2}{{\text{H}}_6}{\text{(g)}}\]
A. –137
B. –33
C. +33
D. +137
The enthalpy change for the dissolution of NH4NO3 is +26 kJ mol–1 at 25 °C. Which statement about this reaction is correct?
A. The reaction is exothermic and the solubility decreases at higher temperature.
B. The reaction is exothermic and the solubility increases at higher temperature.
C. The reaction is endothermic and the solubility decreases at higher temperature.
D. The reaction is endothermic and the solubility increases at higher temperature.
Which equation represents the standard enthalpy of formation of liquid methanol?
A. \({\text{C(g) + 2}}{{\text{H}}_{\text{2}}}{\text{(g) + }}\frac{1}{2}{{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(l)}}\)
B. \({\text{C(g) + 4H(g) + O(g)}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(l)}}\)
C. \({\text{C(s) + 4H(g) + O(g)}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(l)}}\)
D. \({\text{C(s) + 2}}{{\text{H}}_{\text{2}}}{\text{(g) + }}\frac{1}{2}{{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(l)}}\)
Which reaction has an enthalpy change equal to the standard enthalpy change of combustion?
A. \({{\text{C}}_3}{{\text{H}}_8}{\text{(g)}} + {\text{5}}{{\text{O}}_2}{\text{(g)}} \to {\text{3C}}{{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{H}}_2}{\text{O(g)}}\)
B. \({{\text{C}}_3}{{\text{H}}_8}{\text{(g)}} + {\text{5}}{{\text{O}}_2}{\text{(g)}} \to {\text{3C}}{{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{H}}_2}{\text{O(l)}}\)
C. \({\text{2}}{{\text{C}}_4}{{\text{H}}_{10}}{\text{(g)}} + {\text{13}}{{\text{O}}_2}{\text{(g)}} \to {\text{8C}}{{\text{O}}_2}{\text{(g)}} + {\text{10}}{{\text{H}}_2}{\text{O(l)}}\)
D. \({{\text{C}}_5}{{\text{H}}_{12}}{\text{(g)}} + {\text{8}}{{\text{O}}_2}{\text{(g)}} \to {\text{5C}}{{\text{O}}_2}{\text{(g)}} + {\text{6}}{{\text{H}}_2}{\text{O(g)}}\)
Which process is endothermic?
A. \({\text{2}}{{\text{C}}_4}{{\text{H}}_{10}}{\text{(g)}} + {\text{13}}{{\text{O}}_2}{\text{(g)}} \to {\text{8C}}{{\text{O}}_2}{\text{(g)}} + {\text{10}}{{\text{H}}_2}{\text{O(g)}}\)
B. \({\text{Na(g)}} \to {\text{N}}{{\text{a}}^ + }{\text{(g)}} + {{\text{e}}^ - }\)
C. \({{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}} + {\text{2KOH(aq)}} \to {{\text{K}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}\)
D. \({\text{N}}{{\text{H}}_3}{\text{(g)}} \to {\text{N}}{{\text{H}}_3}{\text{(l)}}\)
Which equation represents the bond enthalpy for the H–Br bond in hydrogen bromide?
A. \({\text{HBr(g)}} \to {\text{H(g)}} + {\text{Br(g)}}\)
B. \({\text{HBr(g)}} \to {\text{H(g)}} + {\text{Br(l)}}\)
C. \({\text{HBr(g)}} \to {\text{H(g)}} + \frac{1}{2}{\text{B}}{{\text{r}}_2}({\text{l)}}\)
D. \({\text{HBr(g)}} \to {\text{H(g)}} + \frac{1}{2}{\text{B}}{{\text{r}}_2}({\text{g)}}\)
The same amount of heat energy is added to 1.00 g of each substance.
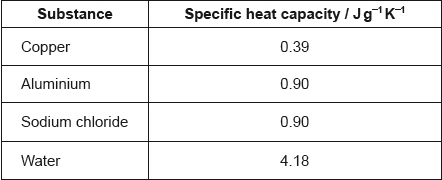
Which statement is correct if all the substances are at the same temperature before the heat energy is added?
A. Copper will reach the highest temperature.
B. Water will reach the highest temperature.
C. All four substances will reach the same temperature.
D. Aluminium will reach a higher temperature than sodium chloride.
1.0 g of sodium hydroxide, NaOH, was added to 99.0 g of water. The temperature of the solution increased from 18.0 °C to 20.5 °C. The specific heat capacity of the solution is \({\text{4.18 J}}\,{{\text{g}}^{ - 1}}{{\text{K}}^{ - 1}}\). Which expression gives the heat evolved in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)?
A. \(\frac{{2{\text{.}}5 \times 100.0 \times 4.18 \times 1000}}{{40{\text{.}}0}}\)
B. \(\frac{{2{\text{.}}5 \times 100.0 \times 4.18}}{{1000 \times 40{\text{.}}0}}\)
C. \(\frac{{2{\text{.}}5 \times 100.0 \times 4.18 \times 40{\text{.}}0}}{{1000}}\)
D. \(\frac{{2{\text{.}}5 \times 1{\text{.}}0 \times 4{\text{.}}18 \times 40{\text{.}}0}}{{1000}}\)
Consider the equations below.
\[\begin{array}{*{20}{l}} {{\text{C}}{{\text{H}}_{\text{4}}}{\text{(g)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{HCHO(l)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}}&{\Delta {H^\Theta } = x} \\ {{\text{HCHO(l)}} + \frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{HCOOH(l)}}}&{\Delta {H^\Theta } = y} \\ {2{\text{HCOOH(l)}} + \frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {{{\text{(COOH)}}}_2}({\text{s)}} + {{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = z} \end{array}\]
What is the enthalpy change of the reaction below?
\[2{\text{C}}{{\text{H}}_4}{\text{(g)}} + {\text{3}}\frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {{\text{(COOH)}}_2}{\text{(s)}} + 3{{\text{H}}_2}{\text{O(l)}}\]
A. \(x + y + z\)
B. \(2x + y + z\)
C. \(2x + 2y + z\)
D. \(2x + 2y + 2z\)
Which processes are exothermic?
I. \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}{\text{(g)}} + {\text{5}}{{\text{O}}_2}{\text{(g)}} \to {\text{3C}}{{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{H}}_2}{\text{O(g)}}\)
II. \({\text{C}}{{\text{l}}_2}{\text{(g)}} \to {\text{2Cl(g)}}\)
III. \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{COOH(aq)}} + {\text{NaOH(aq)}} \to {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{COONa(aq)}} + {{\text{H}}_2}{\text{O(l)}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Which equation represents the standard enthalpy change of formation, \(\Delta H_f^\Theta \), of tetrachloromethane?
A. \({\text{C(g)}} + {\text{4Cl(g)}} \to {\text{CC}}{{\text{l}}_{\text{4}}}{\text{(g)}}\)
B. \({\text{C(s)}} + {\text{4Cl(g)}} \to {\text{CC}}{{\text{l}}_{\text{4}}}{\text{(l)}}\)
C. \({\text{C(g)}} + {\text{2C}}{{\text{l}}_{\text{2}}}{\text{(g)}} \to {\text{CC}}{{\text{l}}_{\text{4}}}{\text{(g)}}\)
D. \({\text{C(s)}} + {\text{2C}}{{\text{l}}_{\text{2}}}{\text{(g)}} \to {\text{CC}}{{\text{l}}_{\text{4}}}{\text{(l)}}\)
Consider the following two equations.
\({\text{2Ca(s)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2CaO(s)}}\) \(\Delta {H^\Theta } = + x{\text{ kJ}}\)
\({\text{Ca(s)}} + {\text{0.5}}{{\text{O}}_2}{\text{(g)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}} \to {\text{CaC}}{{\text{O}}_3}{\text{(s)}}\) \(\Delta {H^\Theta } = + y{\text{ kJ}}\)
What is \(\Delta {H^\Theta }\), in kJ, for the following reaction?
\({\text{CaO(s)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}} \to {\text{CaC}}{{\text{O}}_3}{\text{(s)}}\)
A. \(y - 0.5x\)
B. \(y - x\)
C. \(0.5 - y\)
D. \(x - y\)
Which ionic compound has the most endothermic lattice enthalpy?
A. Sodium chloride
B. Sodium oxide
C. Magnesium chloride
D. Magnesium oxide
The combustion of glucose is exothermic and occurs according to the following equation:
C6H12O6 (s) + 6O2 (g) → 6CO2 (g) + 6H2O (g)
Which is correct for this reaction?
B. 2 × (−394) + (−572) − (−2602)
C. 2 × (−394) + \(\frac{1}{2}\) (−572) + \(\frac{1}{2}\) (−2602)
D. 2 × (−394) + (−572) + (−2602)